Recently, Professor Meng Gu (Department of Materials Science and Engineering), cooperated with Professor Lang Chen (Department of Physics) at SUSTech and Professor Xueliang Sun at the University of Western Ontario, made a series of research progress in alkali metal-air nanobatteries. Relative results were published by Angewandte Chemie International Edition, ACS Nano, and Energy Storage Materials.
Many researchers are devoted to inventing a green batteries system to replace normal lithium-ion batteries. While the alkali metal-air batteries system has a higher theoretical energy density by contrast with Li-ion batteries, it has the possibility to become the next generation power batteries system.
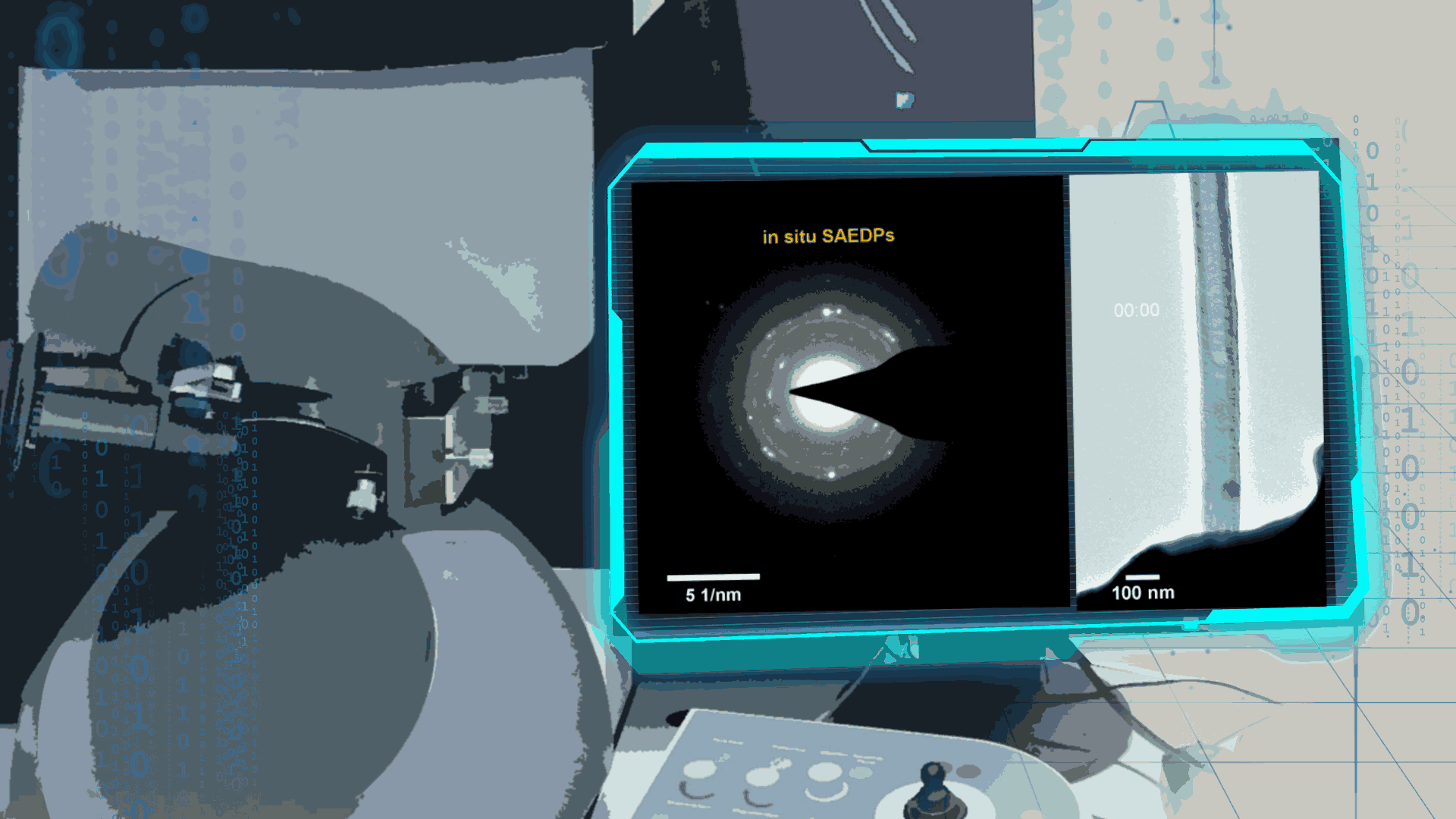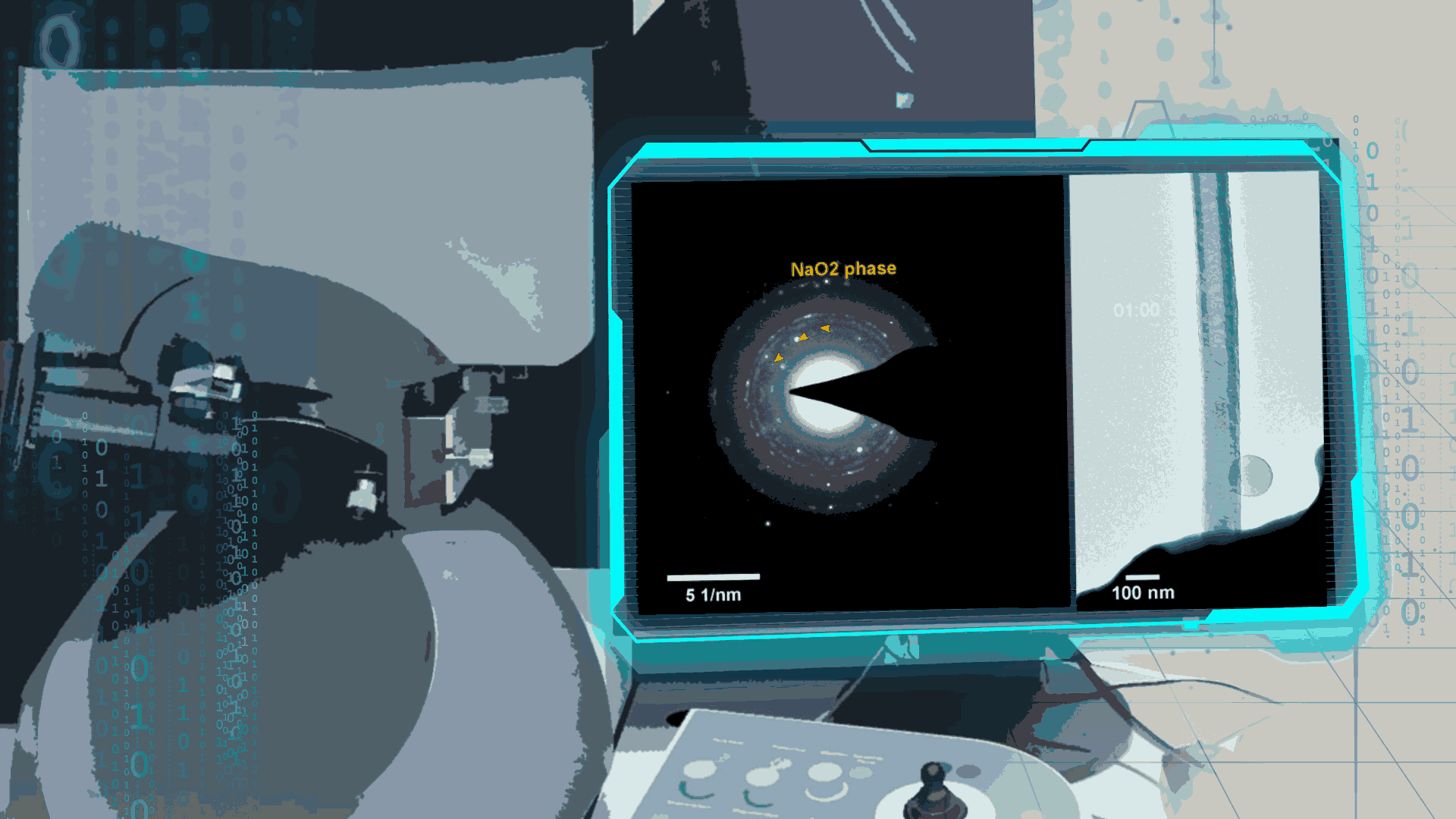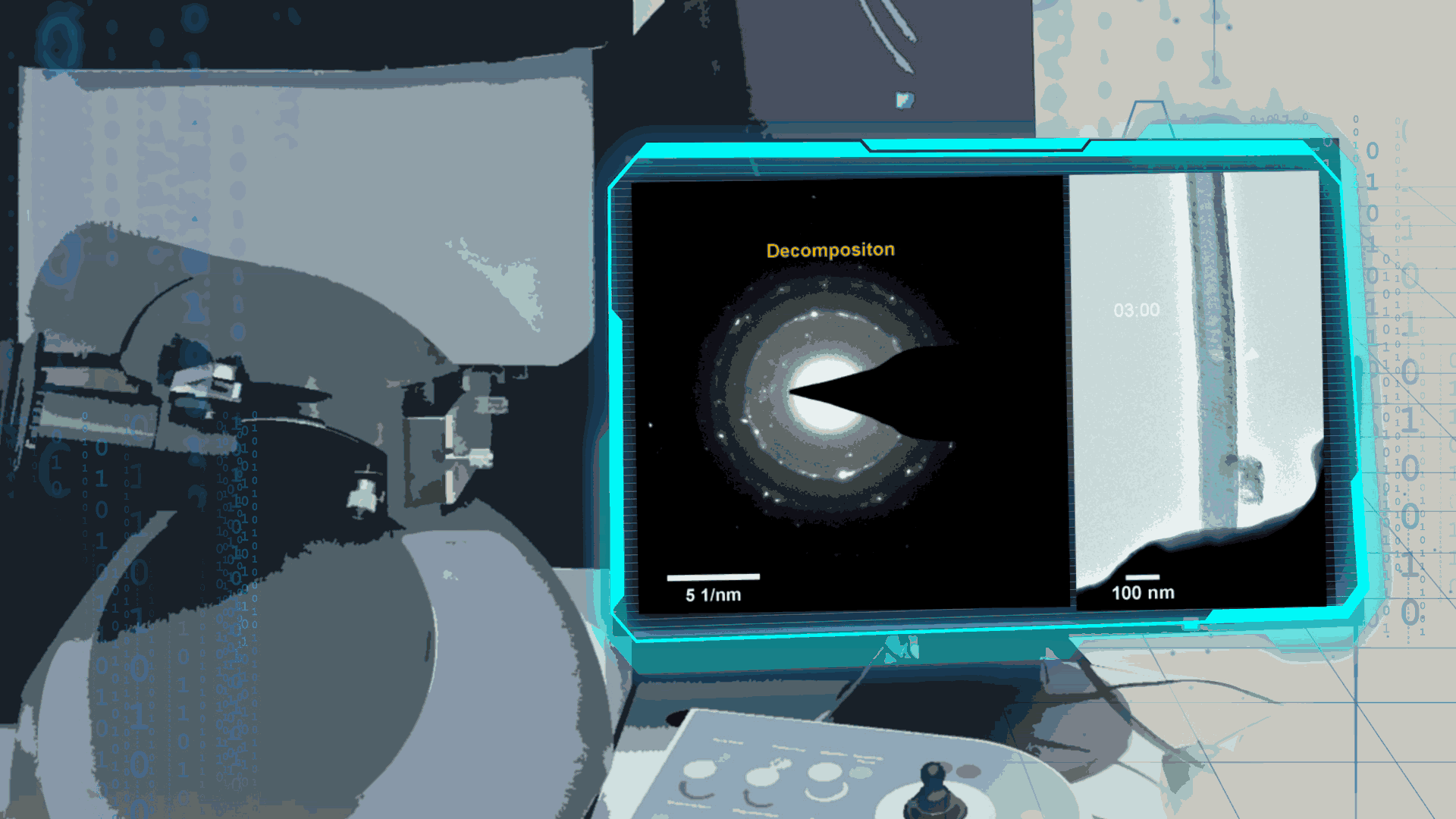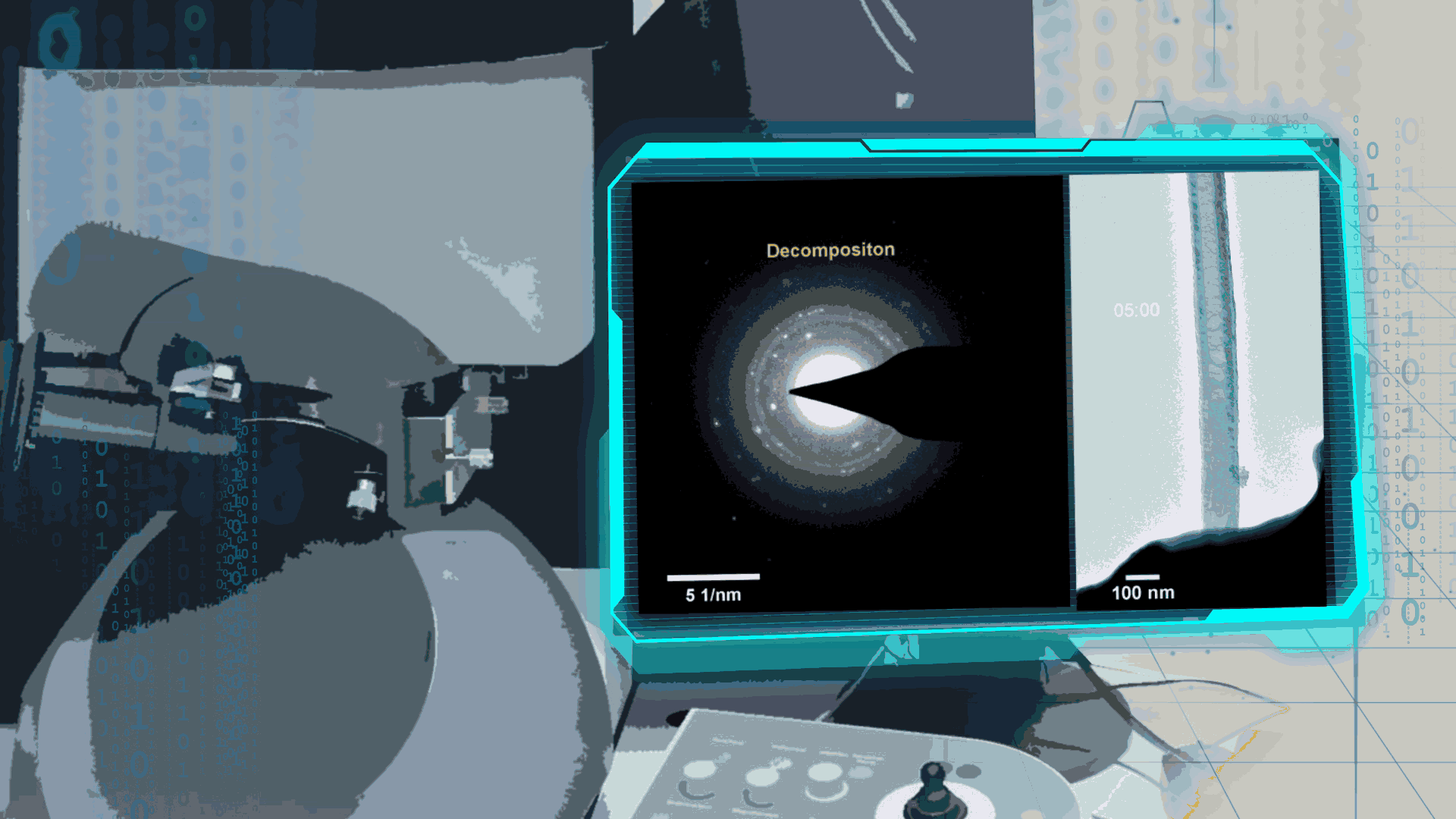
With the development of advanced transmission electron microscopy (TEM) and the appearance of Environment TEM (ETEM), it can observe the growth of materials and structure changes in the specific atmosphere by the researchers. It is also possible to quantitatively analyze the complicative electrochemical reactions at the internal nanobatteries in their different states, such as electricity, heat, force, and gases in ETEM. Using the ETEM, the researchers can directly observe the dynamic reactions in the nanoscale batteries and get the key information, which is the significant method our group used. It is clearer to reveal the progress and mechanism of electrochemical reactions in the nanobatteries, compared to the other methods.
Angew. Chem.: Hybrid electronic and ionic conductor to enhance the dynamics of solid-state Li-air batteries
Solid-state Li-O2 batteries possess the ability to deliver high energy density with enhanced safety. However, the challenge to design a high-functioning solid-state air electrode becomes the main bottleneck for its further development due to the presence of multiple solid-state interfaces, limiting the electron/ion exchange and resulting in poor electrochemical performance. Herein, we propose to adopt a hybrid electronic and ionic conductor to build a solid-state air electrode that makes the transition of Li-O2 battery electrochemical mechanism from a three-phase process to a two-phase process. As a result, the solid-state Li-O2 battery with this hybrid conductor solid-state air electrode exhibits dramatically decreased interfacial resistance and significantly enhanced reaction kinetics. More importantly, the Coulombic efficiency of the Li-O2 battery also significantly improved, benefiting from the good contact between discharge products and electrode materials. In-situ environmental transmission electron microscopy under an oxygen ambiance was used to vividly illustrate the reversible deposition and decomposition of discharge products on the surface of this hybrid conductor, visually verifying the fact of the two-phase reaction.
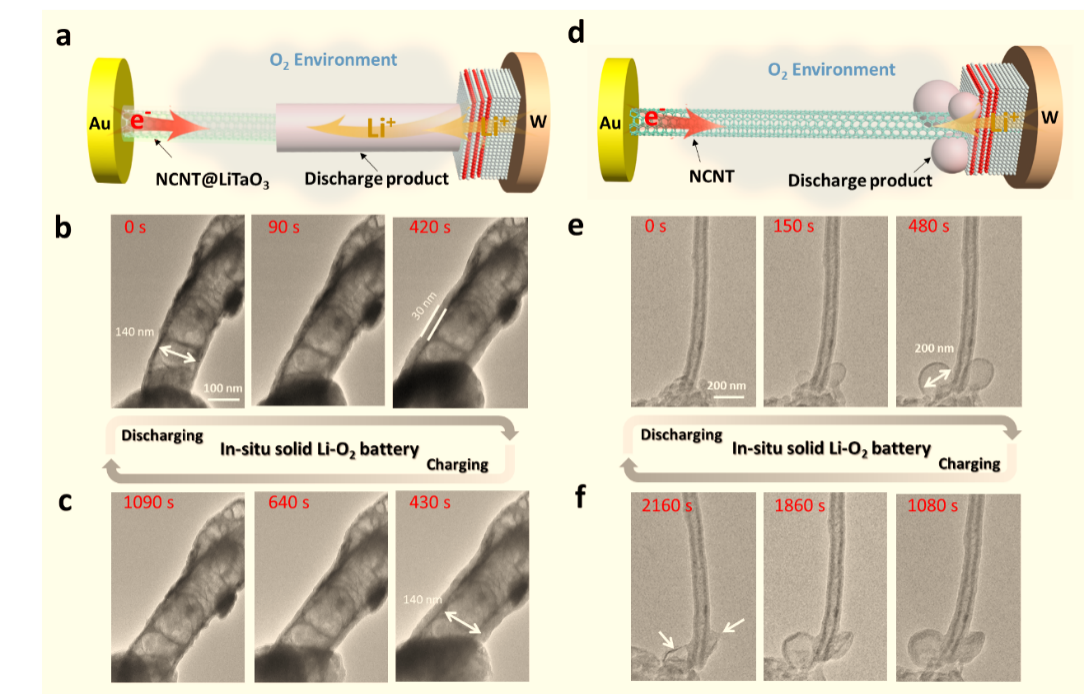
Figure 1. Comparisons of the charge-discharge process with the different cathode of the Li-O2 battery in ETEM.

Figure 2. In-situ observation of the dynamic charge-discharge process of Li-O2 battery on the solid-state hybrid electrode (NCNT@LiTaO3)
The first authors of the paper are Dr. Changtai Zhao of the University of Western Ontario and Dr. Yuanmin Zhu of SUSTech. Professor Meng Gu and Professor Xueliang Sun are corresponding authors.
ACS Nano: In-situ investigation of the charge-discharge behavior and reaction mechanism of cathode supported by alloy catalyst in Na-O2 battery
Compared to lithium-oxygen batteries, sodium–oxygen (Na–O2) batteries exhibit a number of advantages: extremely low cost, low charging overpotential, and stability under nitrogen. However, accumulation of insoluble discharge products and failure of catalysts often result in poor performance of Na–O2 batteries and limit their cycling life. In this work, electrochemical reactions of Na–O2 batteries were directly investigated in situ by assembling a solid-state Na–O2 nanobattery in an aberration-corrected environmental transmission electron microscope. During discharge, NaO2 hollow spheres formed and expanded continuously, accompanying their partial decomposition into Na2O2. These spheres shrank and collapsed into Na2O2 nanoparticles during the charging process. Carbon nanotubes doped with Pt and bimetallic Pt/Ir nanoscale catalyst can promote product formation and reversible evolution. In-depth investigation of the electrochemical reaction mechanism in Na–O2 cells helps to accelerate the development of metal-air devices.

Figure 3. In-situ observation of electrochemical process in Na-O2 nanobatteries by ETEM
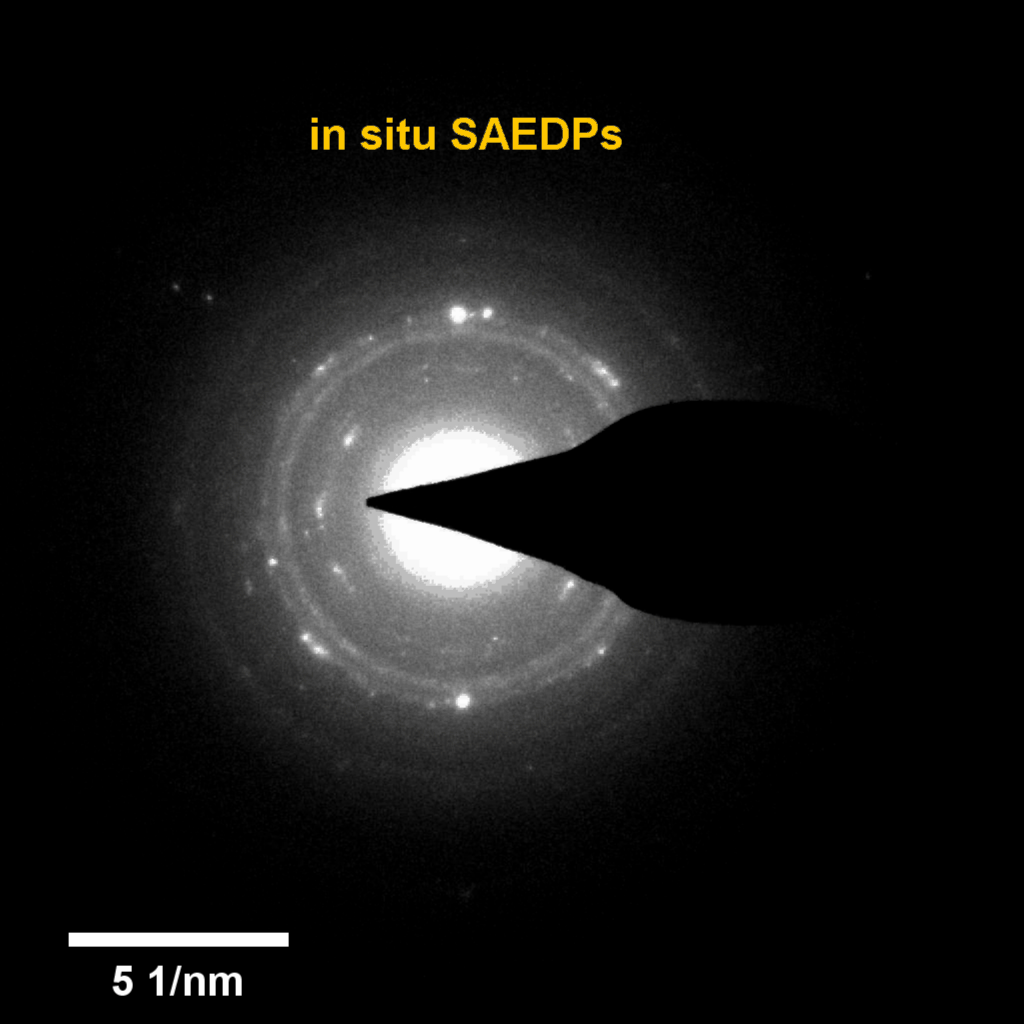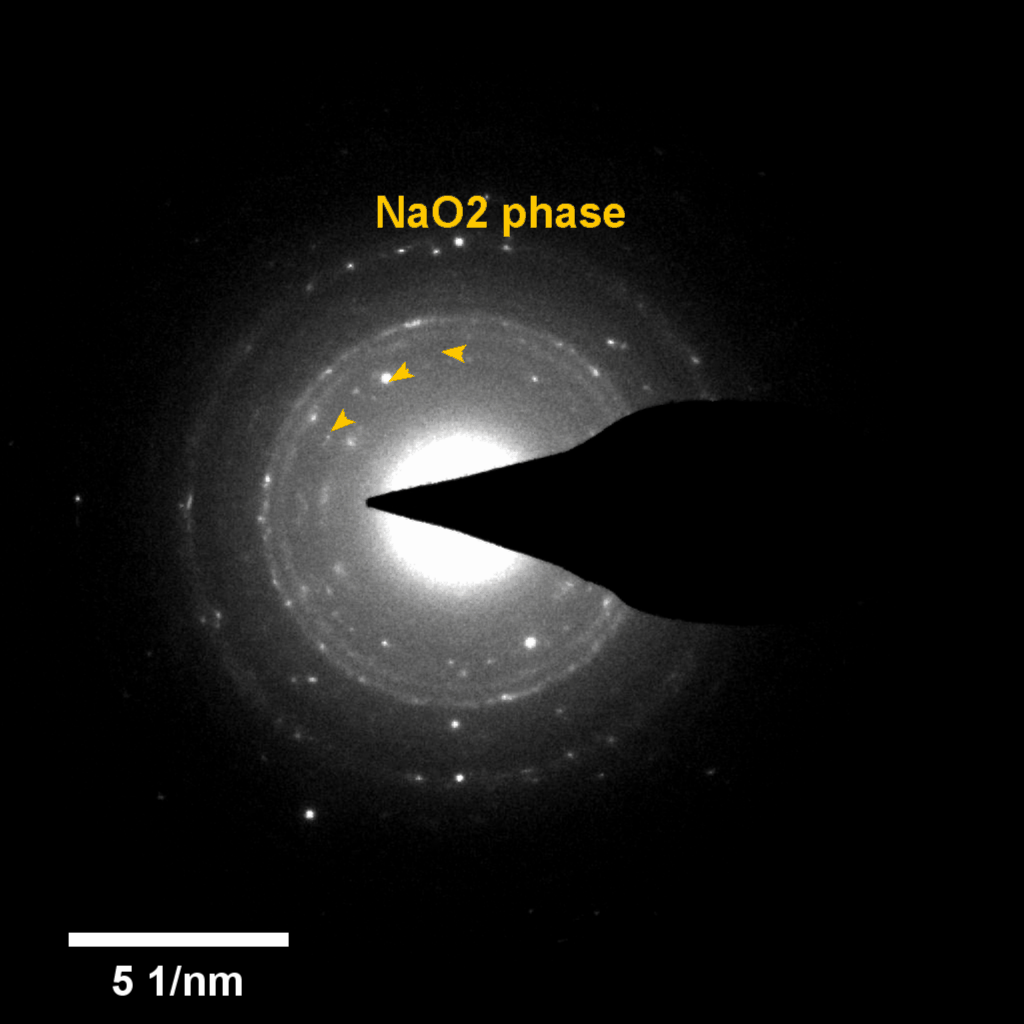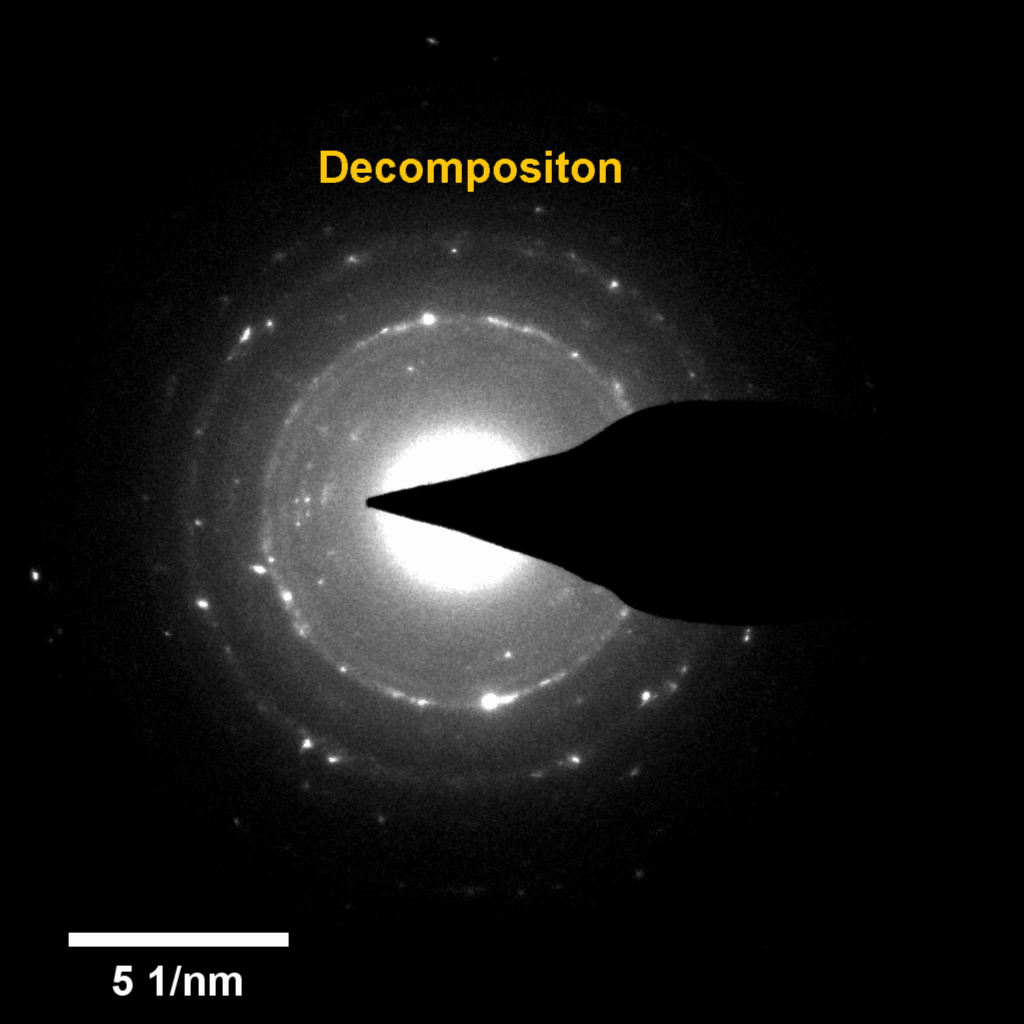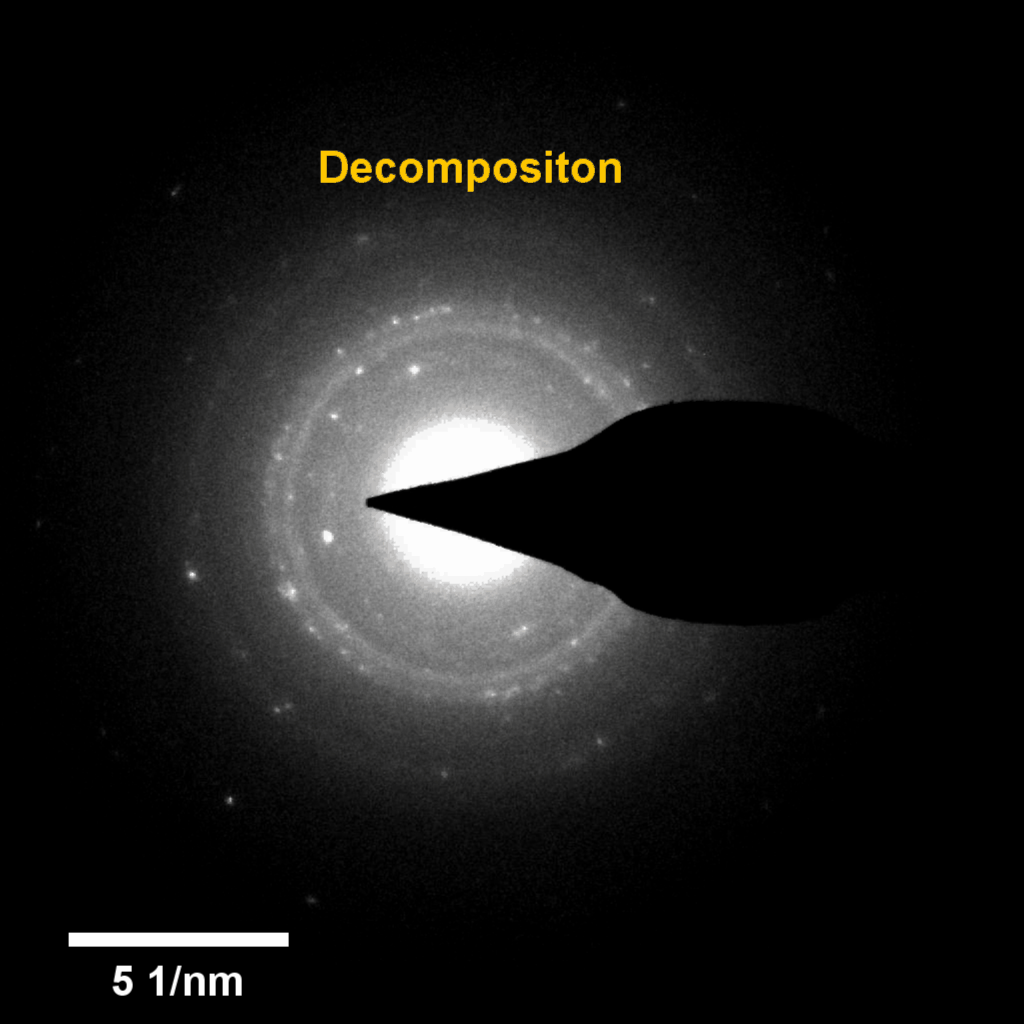
Figure 4. In-situ SAED to dynamically track the microstructural changes process of discharge products
Yuanmin Zhu, a research assistant professor at SUSTech, is the first author of the paper, and Professor Meng Gu is the corresponding author.
Energy Storage Mater.: In-situ investigation of the microevolution mechanism in Na-CO2 batteries catalyzed by a single atom doped catalyst
Na–CO2 batteries possess many virtues including low cost, abundant sodium-containing resources, and environment-friendly nature. Understanding the electrochemical reaction processes is fundamental for battery design and performance enhancement of Na–CO2 batteries. Using in-situ environmental transmission electron microscopy in CO2 gas, we directly probed the morphology evolution and phase transformations of the charge and discharge products with a single Pt atom and nitride doped carbon nanotube (Pt@NCNT) cathode in a Na–CO2 nanobattery. The discharge reaction produced Na2CO3 and carbon, which subsequently decomposed into Na ions and CO2 during charge. The discharge rate was boosted with the help of the single-atom Pt catalyst. Our work provides a fundamental insight into the governing principles on Na–CO2 battery design for better energy storage devices.
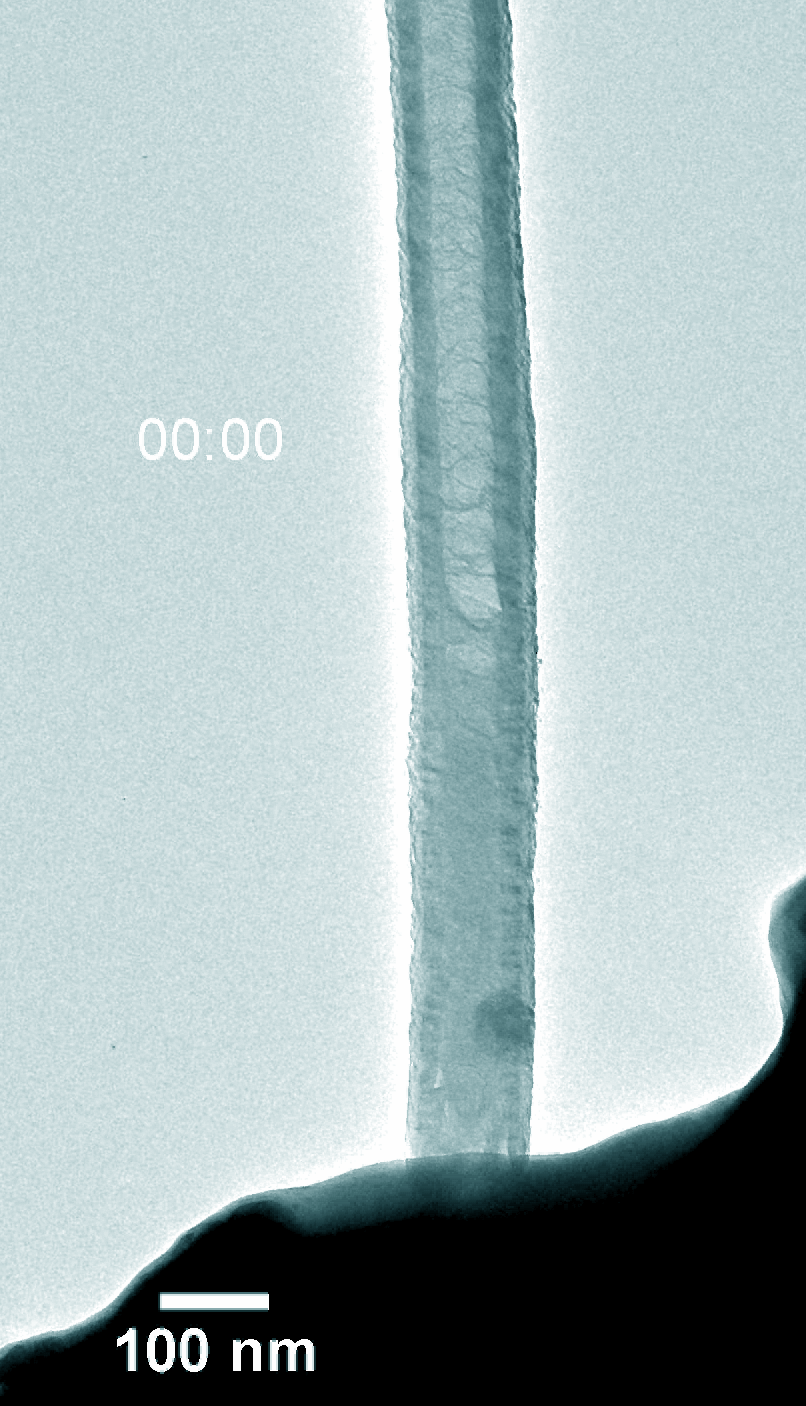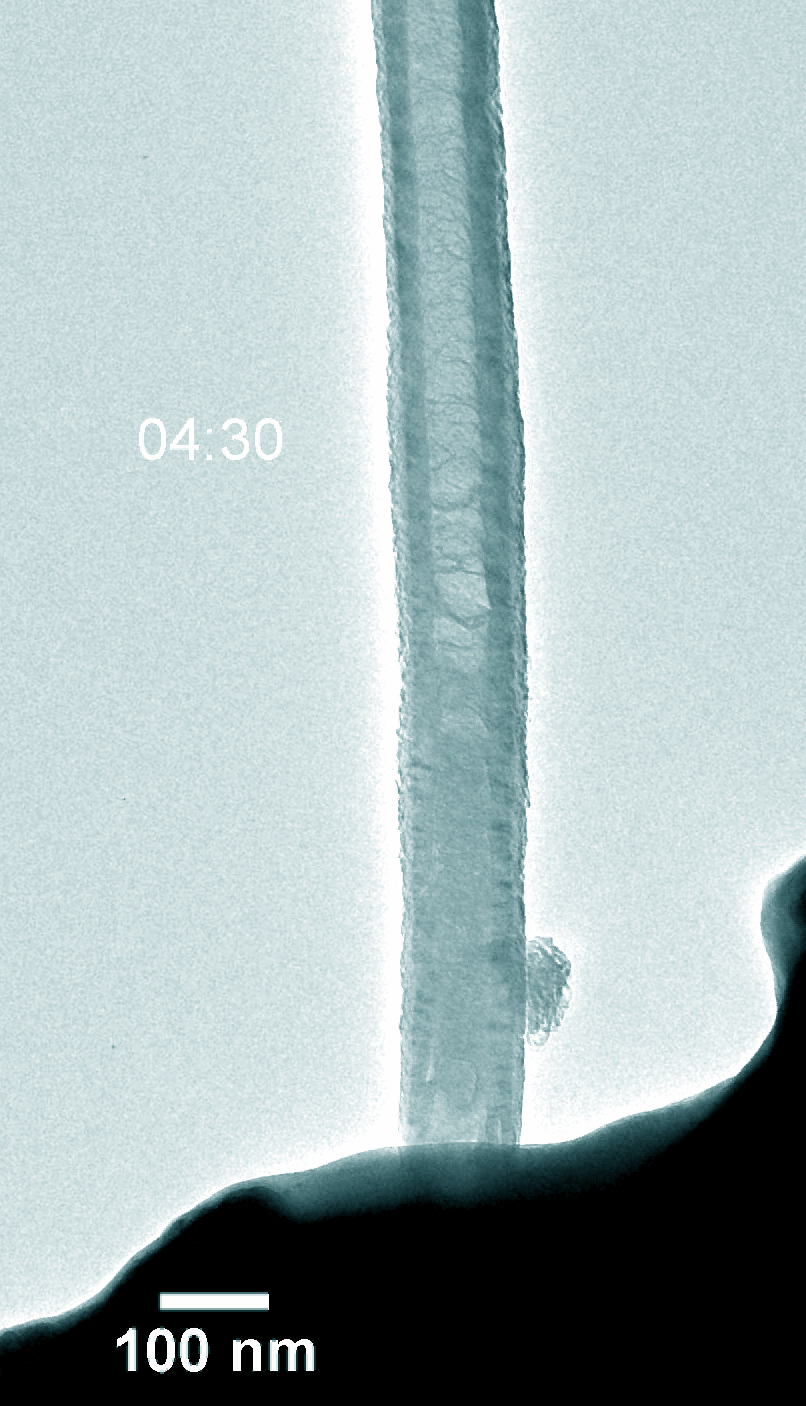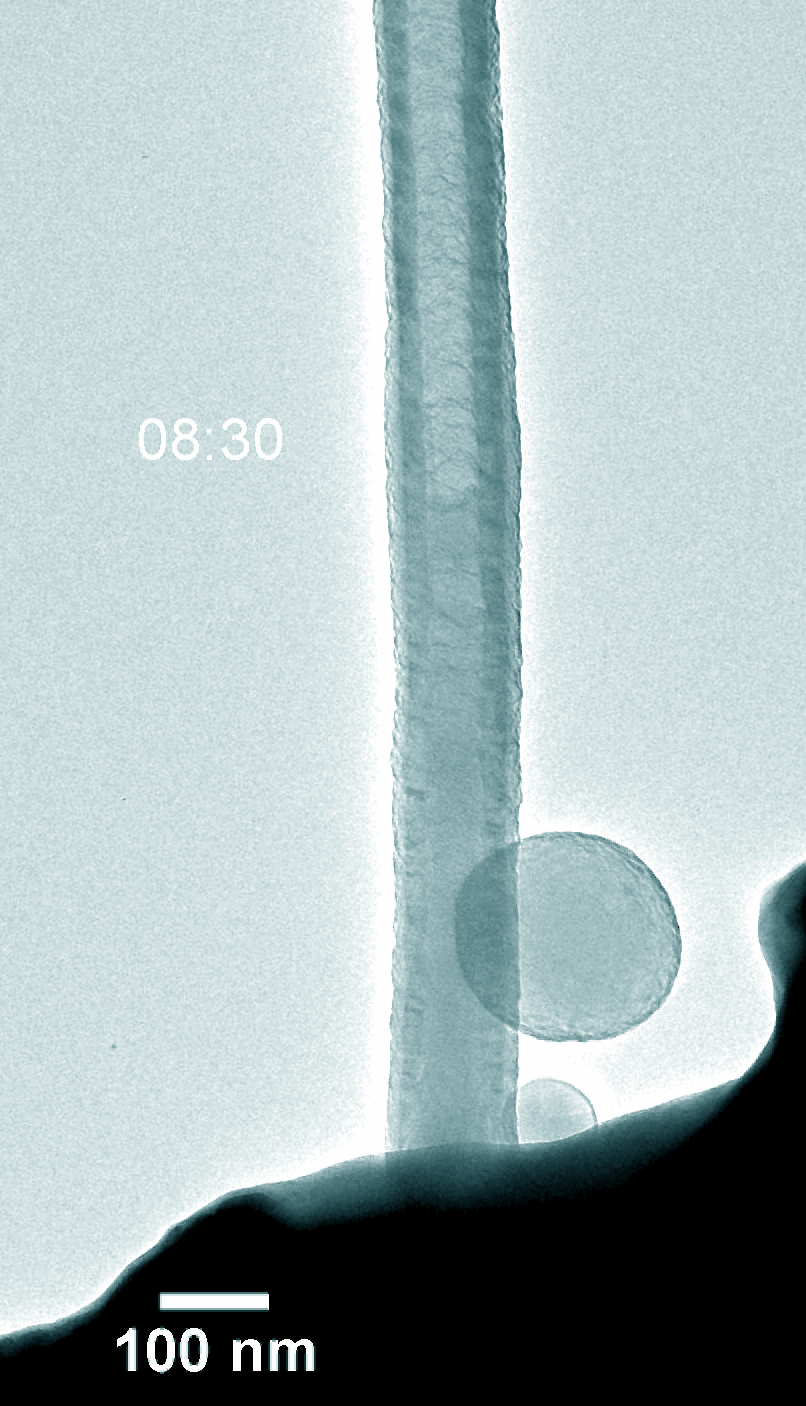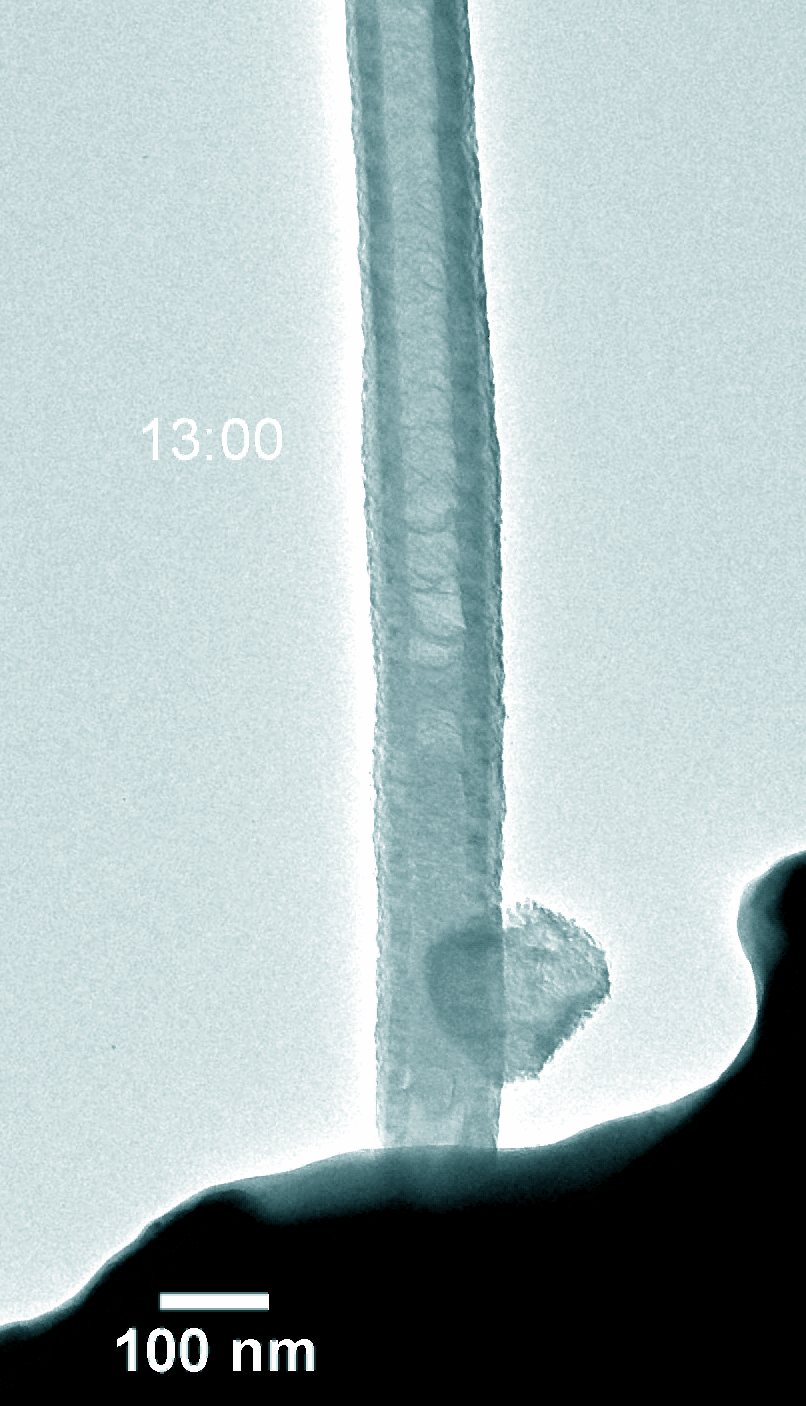
Figure 5. Dynamic evolution of Pt@NCNT in the charge and discharge process.
Dr. Yuanmin Zhu and Shihui Feng at SUSTech are the lead authors of the paper and Professor Meng Gu is the corresponding author.
Paper links:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202014061






