On August 20, Professor Xugang GUO (Materials Science and Engineering) published a Comment in the high-impact academic journal, Nature Materials (IF = 38.663), with the Chief Technology Officer Dr. Antonio Facchetti in Flexterra, Inc. Their Comment, titled “The journey of conducting polymers from discovery to application,” examined the progress of conducting polymers over the 20 years since the groundbreaking accomplishments of Shirakawa, MacDiarmid, and Heeger in 1977. Nature Materials published an Editorial on this topic titled “Conducting polymers forward.”
The field of conducting polymers originated from serendipitous discovery. A student in Japanese Professor Hideki Shirakawa group used far more of a catalyst (a 1000 times as much) than typically used when he was preparing polyacetylene. The polymerization resulted in an unexpected silvery polymer film (Figure 1) on the walls of the reaction vessel, which mystified Shirakawa. Professor Alan G. MacDiarmid was researching unusual conducting inorganic polymers at the same time.
Professor Shirakawa and McDiarmid met at a conference in Tokyo and talked about their findings. While they were unfamiliar with each other, they were attracted to each other’s outcomes. McDiarmid soon invited Shirakawa to the University of Pennsylvania to collaborate with him and colleague Professor Alan J. Heeger, a physicist. By 1977, the trio dramatically improved on the conductivity of trans-polyacetylene after doping with halogens, which led to the world’s first all-organic conducting polymer. Their paper was published in the Journal of the Chemical Society, Chemical Communications (J. Chem. Soc., Chem. Commun.). It shocked the world as it was the first polymer that has a conductivity similar to that of metal.
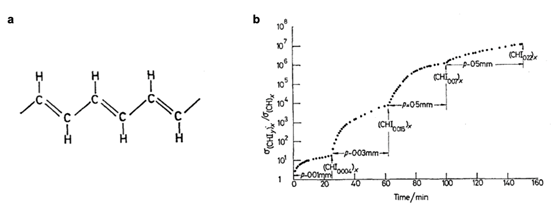
Figure 1. (a) The chemical structure and (b) conductivity of trans-polyacetylene after doping. The conductivity has been improved by ten million times after doping, thus realizing the transition from insulator to conductor
There has been explosive progress in conducting polymers since the awarding of the Nobel Prize in Chemistry in 2000 to three scientists, and the finding also founded the field of organic electronics. Their discovery has led to the commercialization of organic light-emitting diodes (OLEDs), which are widely used in mobile phones and advanced television technology. Material scientists, applied physicists, and electrical engineers have all examined their findings in progressing the discipline.
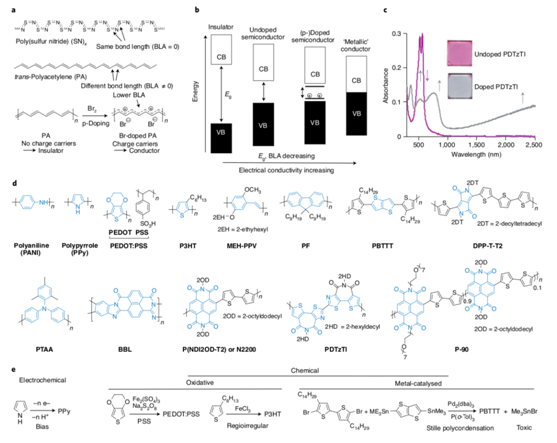
Figure 2. Doping, chemical structure, and synthesis of conducting polymers
Before the work of the Nobel Laureates, there was a prototypical conducting polymer, specifically an inorganic polymer. The inorganic polymer employs poly sulfur nitride, (SN)x (Figure 2), which exhibited an inherent metal conductivity. The seminal discovery by Professor Hideki Shirakawa found that doping the polymer can reduce the energy gap between the top of the valence band and the bottom of the conduction band. It also enabled the polymer with the characteristics of a conductor.
Applications and commercialization efforts
There are significant commercial applications for conducting polymers together with semiconducting polymers. Their uses vary depending on their processing characteristics, the doping (charge density) level, redox properties, and the charge transport characteristics (purely electronic or mixed ionic/electronic). They can be used as antistatic or electromagnetic shielding. It has also been considered for electrode materials in commercial lithium-ion batteries. However, the high cost of conducting polymers has prevented its commercialization at this point.
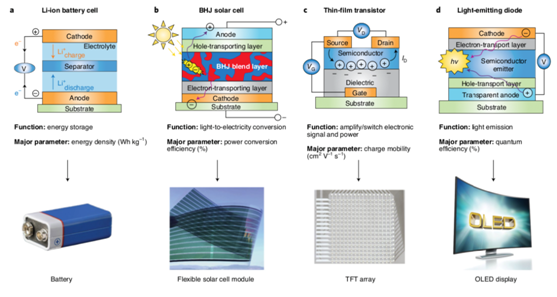
Figure 3. Applications of conducting polymers
Industrial uses for conducting polymers with huge markets include polymer solar cells (PSCs), thin-film transistors for displayers and logic circuits, and OLEDs for display and lighting (Figure 3). It can also be used in perovskite solar cells. However, considerable research is needed in this emerging technology to determine its commercial prospects.
Future
The field of conducting polymers will continue to grow via three related activities: (1) new molecular design and chemistries; (2) fundamental studies refining materials understanding and discovering new phenomena; and (3) exploration of new or revisited fields of use for commercialization.
Advancements in conducting polymers will require the development of effective and green synthesis methods that avoid toxic reagents and minimize by-products, improving economic and environmental sustainability (Figure 4). More progress will come from further fundamental researches into the charge transport at the molecular and supramolecular levels. There are also bioelectronic applications for those conducting polymers with excellent biological compatibility and mechanical flexibility.
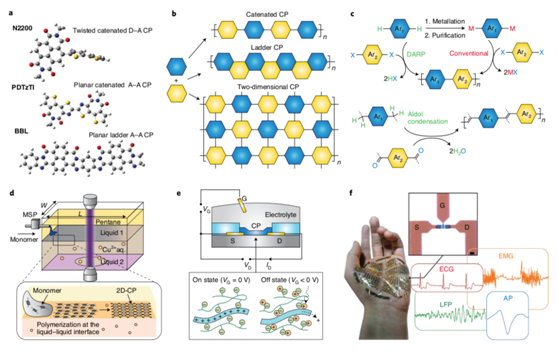
Figure 4. Ongoing fundamental studies and exploration of applications of conducting polymers
The conducting polymer field has undergone dramatic progress from the discovery and seminal studies by the Nobel Laureates to present-day activity.
Pioneering studies taught us the fundamental properties of conducting polymers. Further research will broaden their structural diversity and field of use. Researchers are now aiming to find both new fundamental insights and commercially viable applications.
They believe that this discovery from the 20th century will carry forward into the 21st century, operating at the forefront of materials science. It will influence other disciplines and industries for many generations to come.
Link to the paper: https://www.nature.com/articles/s41563-020-0778-5
Link to the Editorial: https://www.nature.com/articles/s41563-020-0792-7






