Recently, Prof. Yongye Liang’s research group successively published 3 research papers and reported their progress on developing molecular fluorophores for bioimaging in the second near-infrared (NIR-II) window.
Fluorescence imaging is a powerful modality for biological research and biomedical applications. Compared with the traditional visible or the first near-infrared window (NIR-I, 750-900 nm), imaging with fluorescence in the NIR-II window (1000-1700 nm) can provide better spatial resolution and deeper tissue penetration. The lack of fluorophores with high brightness and biocompatibility is a bottleneck for the adoption of bioimaging in the NIR-II window. In previous studies, Liang’s group have developed a shielding unit-donor(s)-acceptor-donor(s)-shielding unit (S-D-A-D-S) structure for NIR-II molecular fluorophores, and synthesized IR-E1 (Adv. Mater., 2016, 28, 6872) and IR-FEP (Adv. Mater., 2017, 29, 1605497) with fluorescent quantum yield (QY) of 0.7% and 2.0% in aqueous solutions. Fluorophores with higher brightness are still needed to achieve ultrafast and high resolution biological imaging.
Liang’s group explored molecular engineering on the donor unit to develop high performance NIR-II fluorophores (Fig. 1). Since the photon scattering in tissue is reduced with increasing wavelength, fluorescence emission at longer wavelength is favored. As a result, a thiophene ring is added as the second donor next to the shielding unit to increase the conjugation length. Such thiophene insertion results in redshift of the absorption and fluorescence emission, but decrease of QYs. The first donor connected to the acceptor is further tuned. Besides oxygen containing EDOT and TEG substituted thiophene, alkyl chain substituted thiophene is also introduced as the first donor. The fluorophore IR-FTAP with octylthiophene as the first donor and thiophene as the second donor shows the optimized performance with fluorescence peaked at 1048 nm and a high QY of 5.3% in aqueous solutions.
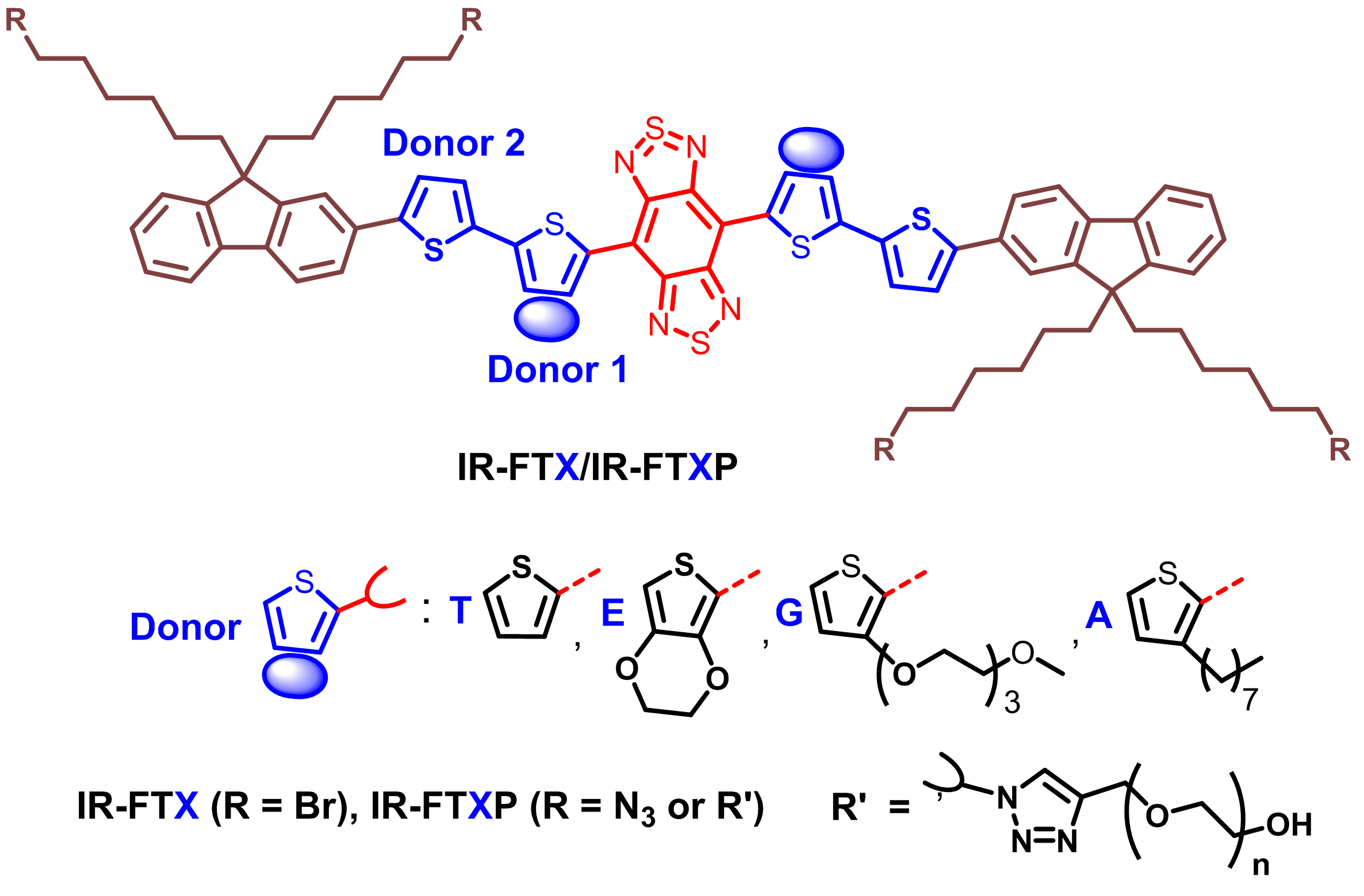
Fig 1.Design of the molecular fluorophores with donor engineering.
The bulky and hydrophobic alkyl thiophene donor affords larger distortion of the conjugated backbone and less interactions with water molecules compared to other donor units studied before, which is confirmed by molecular dynamics simulation and density functional theory calculations (Fig. 2).
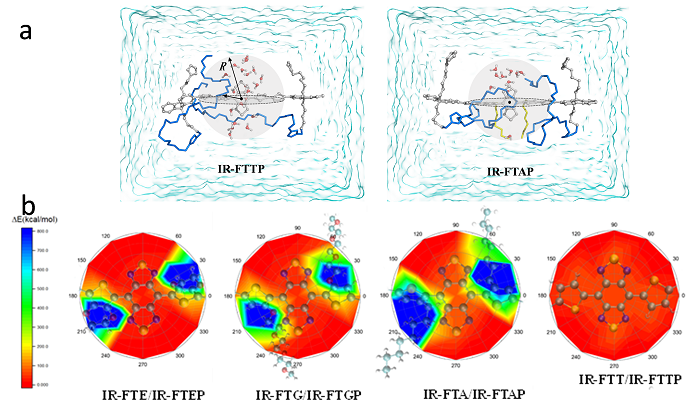
Fig. 2. (a) Representative schematic diagram of water molecules surrounding the BBTD acceptor of the IR-FTAP and IR-FTTP. (b) Schematic illustration of the molecular fluorophores’ interactions with a water molecule. The water molecule is placed 2 Å above the BBTD plane.
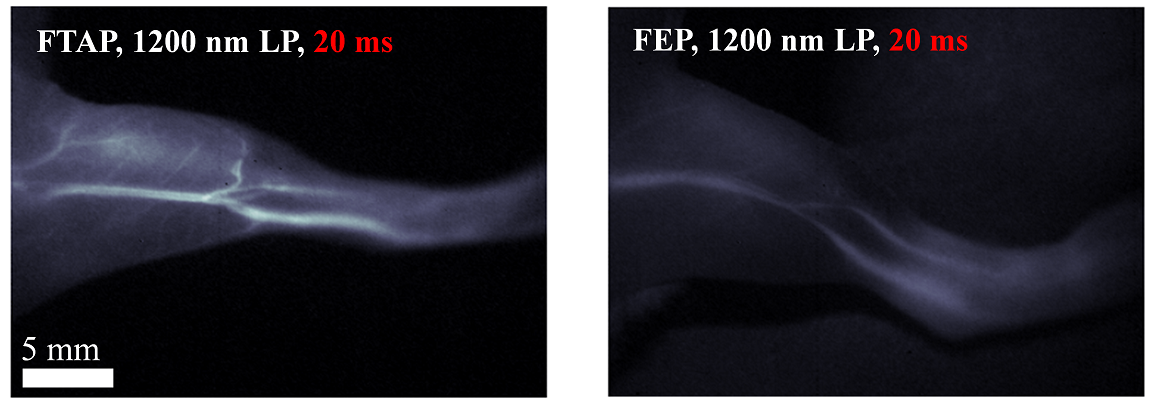
Fig. 3. The hindlimb vessel imaging of IR-FTAP vs IR-FEP.
The high fluorescent performance of IR-FTAP allows for fast (>25 frames s-1) biological imaging of the blood vessels of a mouse hindlimb in the NIR-II window with superior spatial resolution (Fig. 3). This work has been published in the journal “Journal of the American Chemical Society” entitled “Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance”.
The oil-soluble NIR-II molecular fluorophores generally exhibit much higher fluorescent QY in nonpolar solvents (like toluene) than their PEGylated counterparts in aqueous solutions. To retain the high QY of the IR-FE dye and obtain a bright NIR-II fluorophore for biological imaging, Liang’s group and their collaborators encapsulated IR-FE in an amphiphilic polymer matrix to make it biocompatible and bright (Fig. 4). Amphiphilic PEG grafted polystyrene (PS-g-PEG) was prepared to encapsulate IR-FE via evaporation induced assembly process. Specifically, the PS backbone formed a hydrophobic core mimicking the toluene environment to retain the high QY of IR-FE and the PEG side chains off the backbone imparted the complex with aqueous solubility and biocompatibility, thus affording a bright NIR-II fluorophore (p-FE) highly soluble in aqueous solutions.
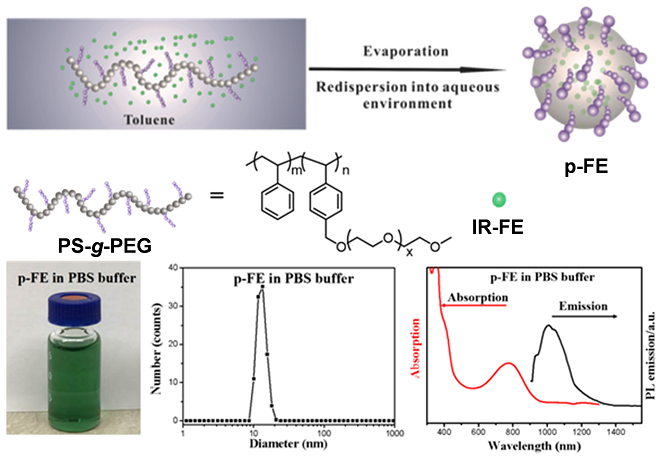
Fig. 4.The schematic representation for the formation of p-FE and its related properties.
p-FE demonstrated superior solubility in PBS, FBS, and cell media. The p-FE nanofluorophore in PBS solution exhibited an absorption peak at ~ 774 nm and emission from 1000 to 1350 nm (peak ~ 1010 nm) under an 808 nm laser excitation (Fig. 1b) with high photo-stability. The relative QY of p-FE in aqueous environment was estimated to be ~ 16.5% based on the IR26 reference fluorophore (QYIR26: ~ 0.5%), which is among the brightest organic molecule based NIR-II fluorophores for biological imaging.
With this bright nanofluorophore, non-invasive ultra-fast in vivo NIR-II imaging of blood flow in the brain vessels of mice with a short exposure time of 2 ms is realized. The bright nanofluorophore enables 3D confocal layer-by-layer imaging of formalin fixed mouse brain tissue to resolve vessels with apparent widths of ~ 5 - 7 µm in diameter at imaging depth up to ~ 1.3 mm in the NIR-II window (Fig. 5), which is the deepest 3D fluorescence microscopy of brain tissues using the one-photon fluorescence technique.
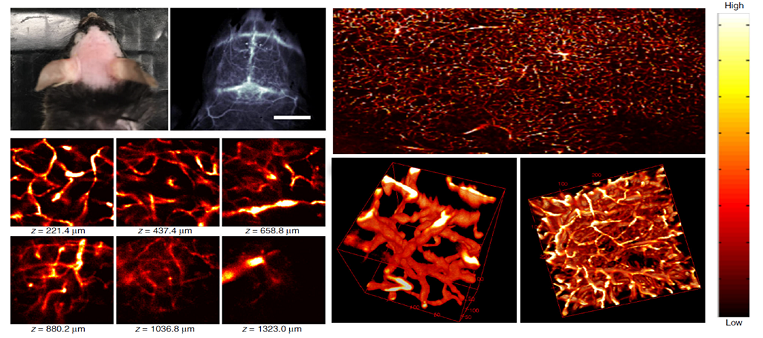
Fig 5. Ex vivo confocal imaging of brain vasculatures of a mouse injected with p-FE.
Liang’s group also introduced PEG on one side chain of IR-FE and carboxyl groups on the other three side chains to yield molecular fluorophore IR-FEPC (Fig. 5). IR-FEPC was further covalently conjugated to the recombinant human chorionic gonadotropin (hCG), a ligand for the luteinizing hormone (LH) receptor. With this probe, their collaborators successfully demonstrated specific imaging of LH receptors in three stages of ovary in vitro and in vivo (Fig. 5). This work has been published in the journal “Advanced Materials” entitled “3D NIR‐II Molecular Imaging Distinguishes Targeted Organs with High‐Performance NIR‐II Bioconjugates”.
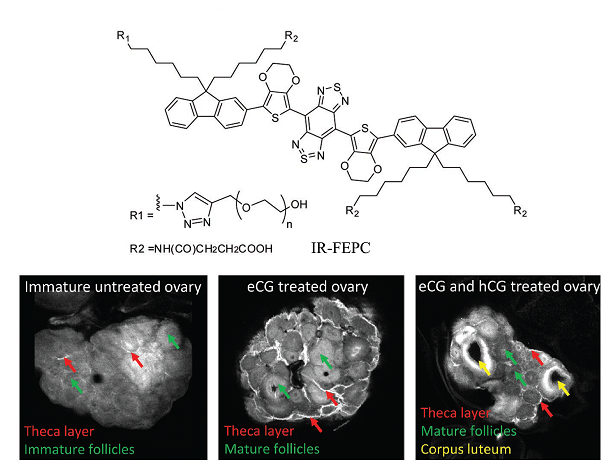
Fig. 6. Molecular fluorophore IR-FEPC and NIR-II imaging of theca layer in ovarian follicles, mature granulosa cells of preovulatory follicles and corpora lutea using hCG@IR-FEPC.
Collaborators for these studies include Prof. Hongjie Dai’s group from Stanford University (bioimaging) and Prof. Haitao Sun’s group from East China Normal University (simulation), and Prof. Xiaodong Zhang’s group from Tianjin University (biocompatibility). These works were supported by “the Shenzhen Key Lab funding (ZDSYS201505291525382), the Shenzhen Technical Research funding (JSGG20160301095829250) and the Shenzhen peacock program (KQTD20140630160825828).
Paper links:
https://pubs.acs.org/doi/abs/10.1021/jacs.7b10334
https://www.nature.com/articles/s41467-018-03505-4
https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201705799






