Recently, the Shenzhen Institute for GU’s lab at Southern University of Science and Technology has made significant experimental progress in oxygen evolution reaction. The research team mainly includes Dr. Chao Cai and Dr. Shaobo Han, students of Prof. Gu, Maoyu Wang, student of Prof. Zhenxing Feng (Organ State University), Meng Gu, Professor of the Institute, and their cooperator, Prof. Feng.
As a fossil energy substitute, hydrogen is characterized by high energy density and zero-emission. Therefore, many approaches are developed to manufacture hydrogens, such as photocatalytic water splitting, biological hydrogen production, and water electrolysis etc.. Benefitting from the low limitations of electricity and high purity of production, the hydrogen from water electrolysis is believed to be the most attractive industrial method. However, the practical application of water electrolysis is limited by the high energy consumption due to the high overpotential of anodic oxygen evolution reaction (OER) and the high cost of the commercial catalysts (noble metal Ru/Ir and their derived oxides). In this sense, developing low-cost catalysts is highly desired for water electrolysis.
Here a new method is developed to prepare single atoms catalysts. The Ir single atoms are loaded onto CoOx amorphous nanosheets (ANSs). This catalyst shows a high OER activity of 160-fold of commercial IrO2 in alkaline solution. The in-situ X-ray absorption spectroscopy (XAS) results disclose the structural evolution of Ir1Co13.3O20.1 ANSs during OER. These results also show the functional modes of synergistic effect between Ir and Co cations during OER. Relevant data are reported on ACS catalysis .
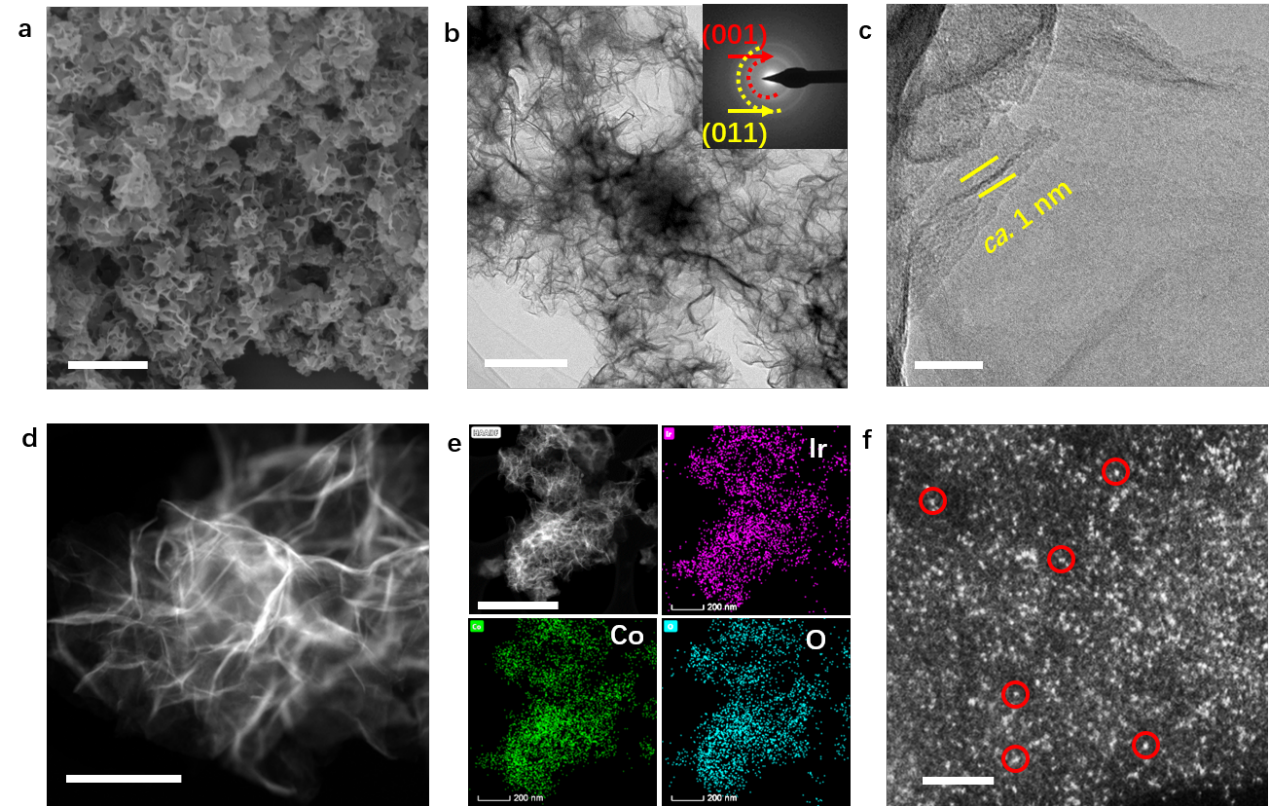
Figure 1. Structural characterization of Ir1Co13.3O20.1. SEM image (a), low-magnification TEM image (b), high-magnification TEM image (c), HAADF image (d), EDS mapping (e), and high-magnification HAADF image (f) of Ir1Co13.3O20.1. Ir single atoms anchor on CoOx ANSs and are well dispersed. The bright spots in Figure 1f correspond to Ir atoms, exemplified by red circles marked. Scale bars in a−f are 1 μm, 200 nm, 20 nm, 100 nm, 500 nm, and 1 nm, respectively.
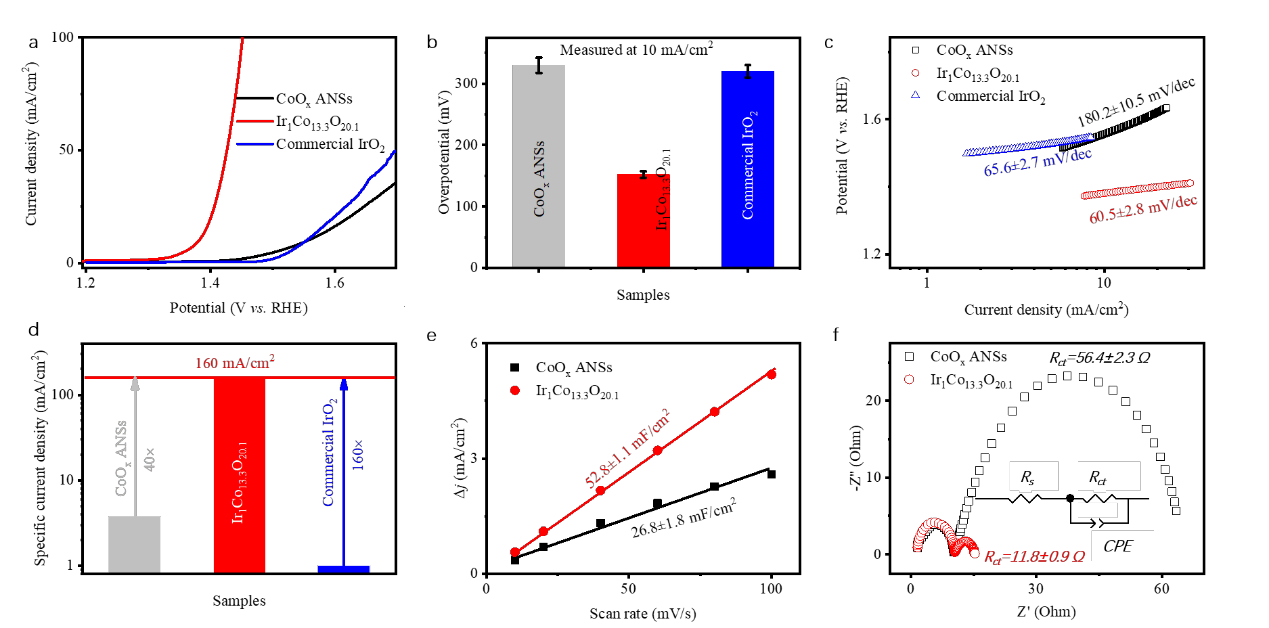
Figure 2. Electrochemical characterizations of different catalysts. Polarization curves (a), collection of overpotential at 10 mA/cm2 (b), Tafel plots (c), specific current density at 1.485 V versus RHE (d), Cdl values (e), and Nyquist plots (f) of CoOx ANSs and Ir1Co13.3O20.1 ANSs. The inset in (f) is the equivalent circuit for Rct calculation. Ir1Co13.3O20.1 ANSs show far better OER performance than CoOx ANSs and IrO2.
The Ir loading can substantially decrease the overpotential as low as 150 mV during OER, which is assisted with the increased electrochemical surface area, charge transport coefficient, energy utilization, and the facilitated O2_ oxidation. Ir1Co13.3O20.1 possesses a low onset potential of <30 mV. The OER occurs on the in-situ formed Ir-O-Co sites, where the O2- near to Ir cations are preferentially oxidized into O2 and gets replenished from the electrolyte. It has advantages in water splitting system and will open up new door for superior catalysts design.
Chao Cai, Maoyu Wang, and Shaobo Han are the co-first authors of the paper. Xiaotao Zu, Zhenxing Feng, and Meng Gu are the corresponding authors. Other authors include Qi Wang, Qing Zhang, Yuanmin Zhu, Xuming Yang, Duojie Wu, and George E. Sterbinsky. This work was supported by National Natural Science Foundation of China, Guangdong Provincial Key Laboratory of Energy Materials for Electric Power with Project No. 2018B030322001, Guangdong Innovative and Entrepreneurial Research Team Program, Shenzhen Peacock Plan Shenzhen DRC project [2018] 1433, and Shenzhen Clean Energy Research Institute.
Paper link: https://pubs.acs.org/doi/10.1021/acscatal.0c04656






