Recently, Prof. Yongye Liang’s research group has developed advanced electrocatalysts for hydrogen evolution reaction (HER) and carbon dioxide reduction by multiscale engineering.
Electrocatalysts play important roles for the applications of renewable energy. However, there are no suitable electrocatalysts with high activities, good stabilities but cost-efficient for many electrochemical conversions.
Recently, molybdenum compounds such as MoS2 have gained wide attention due to their high intrinsic activities in catalyzing HER. To date, the catalytic performance of MoS2 in acidic media has been substantially improved. In contrast, the HER activities of MoS2-based materials in neutral or alkaline media remain low. They are limited by the formation of key intermediate Had in alkaline and neutral HER, which requires the dissociation of water molecule. In this regard, Liang’s group reports an interface engineering method to enhance the HER activities of metallic 1T-MoS2 nanosheets in alkaline and neutral conditions by hybridization with Ni2+δOδ(OH)2−δ nanoparticles (Figure 1). Their results reveal that Ni2+δOδ(OH)2−δ nanoparticles with optimum binding strength to H2O and OHad facilitate the adsorption and dissociation of H2O to supply protons, while 1T-MoS2 nanosheets contribute to the electron transfer, adsorption, and combination of H intermediates and desorption of H2 through a Heyrovsky step (Figure 1). This work has been recently published in the journal “Advanced Science” entitled “Nickel Hydr(oxy)oxide Nanoparticles on Metallic MoS2 Nanosheets: A Synergistic Electrocatalyst for Hydrogen Evolution Reaction”.
http://onlinelibrary.wiley.com/doi/10.1002/advs.201700644/full。
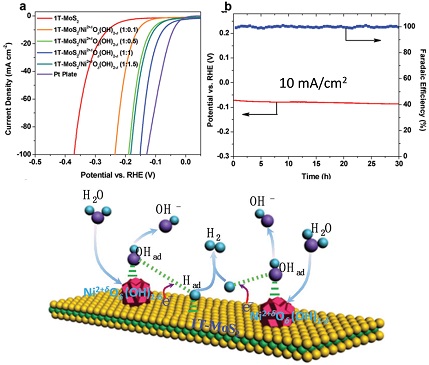
Figure 1. The alkaline HER performance of 1T-MoS2/Ni2+δOδ(OH)2−δ hybrid and the HER mechanism occurred on 1T-MoS2/Ni2+δOδ(OH)2−δ hybrid in alkaline or neutral media
On the other hand, MoP has also recently been found to be active and stable in catalyzing HER in both acidic and alkaline media. However, the syntheses of MoP generally require high temperature, which results in large particle size and low density of surface active sites. Thus, Liang’s group reported a facile method to synthesize an MoP/carbon nanotube (CNT) hybrid featuring small-sized and well-crystallized MoP nanoparticles uniformly coated on the sidewalls of multiwalled carbon nanotubes (CNTs) (Figure 2). CNT can significantly reduce the agglomeration and sintering of MoP nanoparticles due to strong interactions between metal ion and oxygen functional groups on oxidized CNT. Compared to bulk MoP, the MoP/CNT-700 hybrid exhibits much better HER activities in pH-universal electrolytes, delivering a cathodic current density of 10 mA cm-2 with a small overpotential of 83, 102, and 86 mV in acidic 0.5 M H2SO4, neutral 1 M phosphate buffer solution, and alkaline 1 M KOH electrolytes, respectively. his work has been recently published in the journal “Advanced Functional Materials” entitled “Molybdenum Phosphide/Carbon Nanotube Hybrids as pH-Universal Electrocatalysts for Hydrogen Evolution Reaction”.
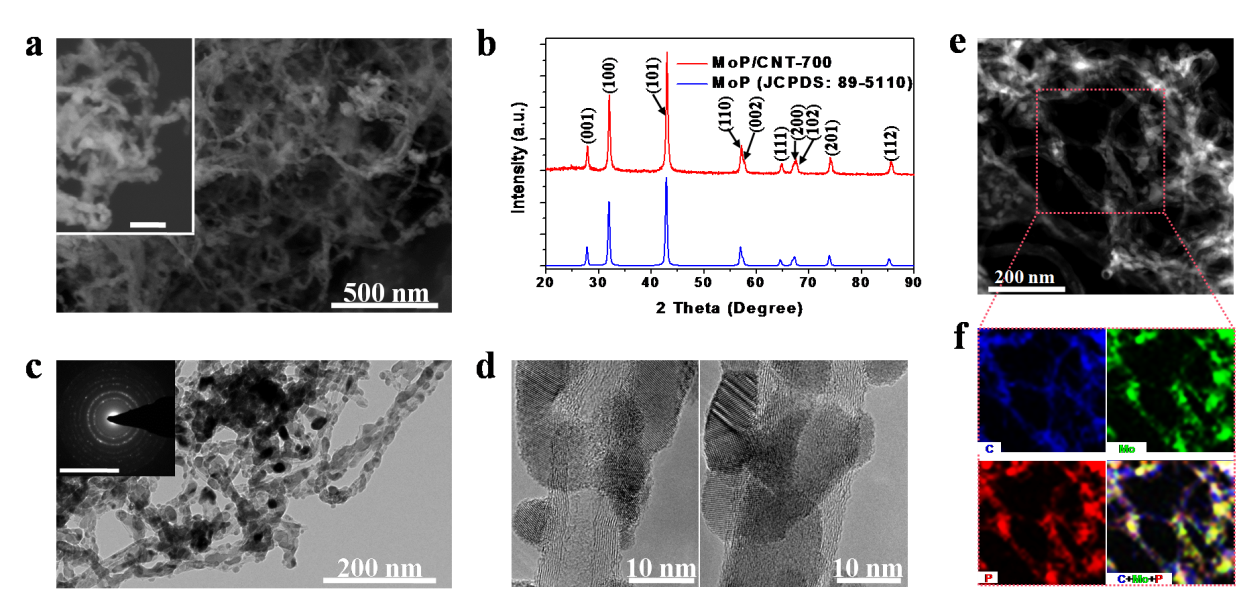
Figure 2. The morphology and structure of MoP/CNT-700 hybrid.
Liang’s group and their collaborators also develop a copper phthalocyanine (CuPc)/CNT hybrid for efficient electrocatalysis of carbon dioxide reduction. The hybrid exhibits by far the highest activity for yielding methane with a Faradaic efficiency of 66% at the potential of – 1.06 V versus the reversible hydrogen electrode (Figure 3). Different with the CoPc/CNT hybrid previously reported by Liang’s group (Nat. Commun. 2017,8,14675), CuPc molecules are dispersed in CNT matrix with nanocrystal aggregation (Figure 3b). By in-situ and operando X-ray absorption spectroscopy, it is found that CuPc molecules restructure to metallic Cu clusters with a size of ~ 2 nm under the working conditions under the working conditions, which catalyzes the carbon dioxide-to-methane conversion (Figure 3c-e). And the Cu nanoclusters convert back to the original CuPc molecules when the negative potential is removed. In contrast, HKUST-1 and [Cu(cyclam)]Cl2 irreversibly decompose to form larger Cu structures, affording much worse catalytic performance. This work has been recently published in the journal “Nature Communications” entitled “Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction”.

Figure 3. a) Faradaic efficiencies of products for CO2 electroreduction reaction catalyzed by CuPc/CNT; b) SEM characterization of CuPc/CNT; c) In situ XAS measurements under electrocatalytic reaction conditions for Cu K-edge XANES spectra; d) First-order derivatives of the XANES spectra before and after electrocatalysis. e) First-shell Cu–Cu coordination numbers of the CuPc catalyst at different potentials.
These work are supported by Shenzhen fundamental research funding (JCYJ20160608140827794) and so on.
Paper links: http://onlinelibrary.wiley.com/doi/10.1002/advs.201700644/full
http://onlinelibrary.wiley.com/doi/10.1002/adfm.201706523/full






